湖南省细菌耐药监测网2012—2021年尿标本分离细菌耐药监测报告
税剑1
,
陈丽华2
,
李晨3
,
李艳明4
,
刘君5
,
宁兴旺6
,
邬靖敏7
,
杨怀德8
,
袁红霞9
,
郑铭10,11,12
,
任南10,11,12,13
,
吴安华10,11,12,13
,
黄勋10,11,12,13
,
付陈超10,11,12


,
石国民1


1. 长沙市中心医院检验科,湖南 长沙 410004;
2. 中南大学湘雅三医院检验科,湖南 长沙 410013;
3. 浏阳市中医医院检验科,湖南 浏阳 410300;
4. 中南大学湘雅医院检验科,湖南 长沙 410008;
5. 湘潭市中心医院检验科,湖南 湘潭 411100;
6. 湖南中医药大学第一附属医院医学检验与病理中心,湖南 长沙 410011;
7. 长沙市第一医院检验科,湖南 长沙 410005;
8. 张家界市人民医院检验科,湖南 张家界 427000;
9. 郴州市第一人民医院检验医学中心,湖南 郴州 423000;
10. 中南大学湘雅医院医院感染控制中心,湖南 长沙 410008;
11. 湖南省细菌耐药监测网办公室,湖南 长沙 410008;
12. 国家老年疾病临床医学研究中心(湘雅医院),湖南 长沙 410008;
13. 湖南省医院感染管理质量控制中心,湖南 长沙 410008
收稿日期:2024-01-22
基金项目:湖南省自然科学基金项目(2021JJ40624);湖南省卫生健康委科研计划项目(202111000032)
作者简介:税剑(1986-), 男(土家族), 湖北省恩施市人, 主管技师, 主要从事临床微生物检验研究.
摘要:目的 了解湖南省临床尿标本细菌分布及耐药情况, 为尿路感染患者的抗感染治疗提供科学依据。方法 按照全国细菌耐药监测网技术方案要求开展细菌耐药监测工作, 参考美国临床实验室标准化协会标准判断药敏试验结果, 应用WHONET 5.6软件分析2012—2021年湖南省细菌耐药监测网成员单位上报的尿标本分离细菌的菌株资料及耐药性数据。结果 2012—2021年尿标本检出细菌379 330株, 其中革兰阴性菌占75.3% (72.4%~76.0%), 革兰阳性菌占24.7%(24.0%~27.6%)。革兰阴性菌中居前5位的细菌分别为大肠埃希菌(48.3%)、肺炎克雷伯菌(8.4%)、奇异变形杆菌(3.3%)、铜绿假单胞菌(3.0%)、阴沟肠杆菌(1.6%)。革兰阳性菌中居前5位的细菌分别为屎肠球菌(8.1%)、粪肠球菌(6.6%)、表皮葡萄球菌(1.9%)、金黄色葡萄球菌(1.9%)、溶血葡萄球菌(1.4%)。不同性别及不同年龄段患者尿标本检出细菌构成不同, 居首位的大肠埃希菌在男性、女性中分别占34.8%、57.2%, 在成人和儿童中分别占49.0%、34.4%。大肠埃希菌对碳青霉烯类抗生素、阿米卡星、替加环素、头孢哌酮/舒巴坦、呋喃妥因保持较高的敏感性, 耐药率<10%;对头孢唑林、头孢呋辛、头孢曲松、头孢噻肟、喹诺酮类抗菌药物的耐药性较高, 耐药率>48%;对头孢他啶、头孢曲松、头孢噻肟、头孢吡肟、氨曲南的耐药率呈下降趋势(均P<0.001)。肺炎克雷伯菌对碳青霉烯类抗生素、阿米卡星、替加环素保持较高的敏感性, 耐药率<11%, 对喹诺酮类抗菌药物耐药率远低于大肠埃希菌。屎肠球菌、粪肠球菌对万古霉素、替考拉宁、利奈唑胺均保持较高的敏感性, 耐药率均<5%;粪肠球菌对氨苄西林、呋喃妥因的耐药率<15%;除利奈唑胺和米诺环素外, 屎肠球菌对被检测抗菌药物的耐药率均高于粪肠球菌。未检出对万古霉素、利奈唑胺、替考拉宁耐药的金黄色葡萄球菌。结论 湖南省细菌耐药监测网各成员单位尿标本检出细菌以大肠埃希菌为主, 在早期经验性治疗的同时, 临床应尽快根据细菌鉴定和药敏结果进行目标用药, 从而提高治疗效果和减缓耐药产生。
关键词:尿路感染 抗菌药物 耐药性 监测 湖南省细菌耐药监测网
Antimicrobial resistance of bacteria isolated from urine specimens: surveillance report from Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
SHUI Jian1
,
CHEN Li-hua2
,
LI Chen3
,
LI Yan-ming4
,
LIU Jun5
,
NING Xing-wang6
,
WU Jing-min7
,
YANG Huai-de8
,
YUAN Hong-xia9
,
ZHENG Ming10,11,12
,
REN Nan10,11,12,13
,
WU An-hua10,11,12,13
,
HUANG Xun10,11,12,13
,
FU Chen-chao10,11,12


,
SHI Guo-min1


1. Department of Laboratory Medicine, Changsha Central Hospital, Changsha 410004, China;
2. Department of Laboratory Medicine, The Third Xiangya Hospital of Central South University, Changsha 410013, China;
3. Department of Laboratory Medicine, Liuyang Traditional Chinese Medicine Hospital, Liuyang 410300, China;
4. Department of Laboratory Medicine, Xiangya Hospital, Central South University, Changsha 410008, China;
5. Department of Laboratory Medicine, Xiangtan Central Hospital, Xiangtan 411100, China;
6. Medical Laboratory and Patho-logy Center, The First Hospital of Hunan University of Chinese Medicine, Changsha 410011, China;
7. Department of Laboratory Medicine, The First Hospital of Changsha, Changsha 410005, China;
8. Department of Laboratory Medicine, Zhangjiajie People's Hospital, Zhangjiajie 427000, China;
9. Center for Laboratory Medicine, The First People's Hospital of Chenzhou, Chenzhou 423000, China;
10. Center for Healthcare-associated Infection Control, Xiangya Hospital, Central South University, Changsha 410008, China;
11. Hunan Province Antimicrobial Resistance Surveillance System Office, Changsha 410008, China;
12. National Clinical Research Center for Geriatric Disorders [Xiangya Hospital], Changsha 410008, China;
13. Hunan Provincial Healthcare-associated Infection Management Quality Control Center, Changsha 410008, China
Abstract: Objective To understand the distribution and antimicrobial resistance of bacteria from clinical urine specimens in Hunan Province, and provide scientific basis for anti-infection treatment of patients with urinary tract infection (UTI). Methods Bacterial resistance surveillance was carried out according to the technical scheme requirements of China Antimicrobial Resistance Surveillance System, antimicrobial susceptibility testing results were judged based on standard of American Clinical and Laboratory Standards Institute, data about strains and antimicrobial resistance of bacteria from urine specimens reported by member units of Hunan Province Antimicrobial Resistance Surveillance System in 2012-2021 were analyzed with WHONET 5.6 software. Results A total of 379 330 strains of bacteria were isolated from urine specimens in 2012-2021, Gram-negative and Gram-positive bacteria accounted for 75.3% (72.4%-76.0%) and 24.7% (24.0%-27.6%), respectively. The top 5 Gram-negative bacteria were Escherichia coli (48.3%), Klebsiella pneumoniae (8.4%), Proteus mirabilis (3.3%), Pseudomonas aeruginosa (3.0%) and Enterobacter cloacae (1.6%). The top 5 Gram-positive bacteria were Enterococcus faecium (8.1%), Enterococcus faecalis (6.6%), Staphylococcus epidermidis (1.9%), Staphylococcus aureus (1.9%) and Staphylococcus haemolyticus (1.4%). The constituent of bacteria isolated from urine specimens of patients in different gender and age groups were different. Escherichia coli ranked first, accounting for 34.8% and 57.2% in males and females, respectively, as well as 49.0% and 34.4% in adults and children, respectively. Escherichia coli maintained high susceptibility to carbapenems, amikacin, tigecycline, cefoperazone/sulbactam and furantoin, with resistance rate < 10%, while resistance to cefazolin, cefuroxime, ceftriaxone, cefotaxime and quinolones were relatively higher, with resistance rate >48%; resistance rates to ceftazidime, ceftriaxone, cefotaxime, cefepime and aztreonam presented decreased trend (all P < 0.001). Klebsiella pneumoniae maintained higher susceptibility to carbapenems, amikacin and tigecycline, with resistance rate < 11%, resistance rate to quinolones was much lower than that of Escherichia coli. Enterococcus faecium and Enterococcus faecalis maintained high susceptibility to vancomycin, teicoplanin and linezolid, with resistance rates < 5%; resistance rate of Enterococcus faecalis to ampicillin and furantoin was < 15%. Except for linezolid and minocycline, resistance rates of Enterococcus faecium to the other tested antimicrobial agents were all higher than Enterococcus faecalis. No Staphylococcus aureus was found to be resistant to vancomycin, linezolid and teicoplanin. Conclusion Escherichia coli is the main bacteria isolated from urine specimens from various member units of Hunan Province Antimicrobial Resistance Surveillance System. In early empirical treatment, clinical antimicrobial should be targetedly used as early as possible based on bacterial identification and antimicrobial susceptibility testing results, so as to improve treatment effectiveness and slow down the emergence of antimicrobial resistance.
Key words:
urinary tract infection antimicrobial agent antimicrobial resistance surveillance Hunan Province Antimicrobial Resistance Surveillance System
尿路感染是医院和社区常见的感染性疾病。近年来随着侵入性操作增多,医院获得性尿路感染也逐年升高,在各种感染性疾病中仅次于呼吸道感染居第2位[1-2],全球每年有近2亿人发生尿路感染,50%~70%的女性一生至少罹患过一次尿路感染。尿路感染若不能得到及时有效的治疗,可导致从简单的膀胱炎发展成严重的脓毒败血症休克,甚至肾衰竭,严重影响患者的生活质量和生命安全。采集中段尿培养获得病原菌,是诊断尿路感染的重要手段,对病原菌进行药敏试验,可以为临床抗感染治疗提供科学依据。目前尿路感染的治疗主要基于抗菌药物的使用,如β-内酰胺类、氨基糖苷类、喹诺酮类和呋喃妥因等,由于这些抗菌药物的大量使用甚至滥用,导致病原菌对这些抗菌药物的耐药率不断升高,给临床抗感染治疗带来严峻挑战[3-5]。为了解湖南省各医院尿路感染患者尿标本分离病原菌的构成及耐药情况,为该省及其他地区尿路感染患者经验性抗感染治疗及医院感染控制管理提供科学依据,本研究回顾性分析2012—2021年10年间湖南省细菌耐药监测网成员单位尿标本分离细菌的构成及耐药情况,现报告如下。
1 资料与方法
1.1 数据来源
尿标本分离病原菌的全部监测数据来自2012—2021年湖南省细菌耐药监测网成员单位。各监测网点医院将细菌监测数据从医院信息系统、药敏测定系统直接导入或手工录入WHONET软件,通过湖南省细菌耐药监测网上报,要求报告细菌药敏的最低抑菌浓度(MIC)值或抑菌圈直径。经数据审核,剔除质量不合格单位,2012—2021年纳入数据分析的医院数分别为162、162、166、164、161、163、163、166、165、162所。
1.2 技术方案
细菌鉴定方法、质控菌株选择及测试抗菌药物种类参照全国细菌耐药监测网(CARSS)技术方案执行[6]。药敏试验结果按照美国临床实验室标准化协会(Clinical & Laboratory Standards Institute, CLSI)推荐的抗微生物药物敏感性试验执行标准2022年版(M100第32版)进行判断[7],结果分为敏感(S)、中介/剂量依赖型敏感(I/SDD)、耐药(R)三种情况,文中I/SDD结果未列出。其中头孢哌酮/舒巴坦无药敏解释折点,参照头孢哌酮折点判断[8-9]。替加环素采用美国食品药品监督管理局(Food and Drug Administration, FDA)推荐的折点[10]。多黏菌素B参考欧盟药敏试验标准委员会(European Committee on Antimicrobial Susceptibility Testing, EUCAST)推荐折点[11]。
1.3 统计分析
依据每例患者相同标本统计第一株菌的原则,剔除重复菌株。应用WHONET 5.6软件进行药敏结果分析。应用SPSS 21.0对耐药率、检出率进行统计学分析,以P≤0.05为差异有统计学意义。
2 结果
2.1 细菌数量、种类
2.1.1 菌株数量
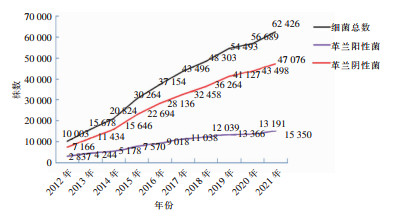
2012—2021年纳入分析的尿标本分离细菌共计379 330株,由2012年的10 003株增加至2021年62 426株,其中革兰阳性菌由2 837株增加至15 350株,革兰阴性菌由7 166株增加至47 076株,均呈逐年增加趋势。见图 1。
2.1.2 菌株构成
2012—2021年尿标本临床分离的细菌以革兰阴性菌为主,占比为75.3%(72.4%~76.0%),革兰阳性菌占比为24.7%(24.0%~27.6%)。革兰阴性菌中居前5位的细菌分别为大肠埃希菌(183 273株,48.3%)、肺炎克雷伯菌(31 754株,8.4%)、奇异变形杆菌(12 446株,3.3%)、铜绿假单胞菌(11 372株,3.0%)和阴沟肠杆菌(5 935株,1.6%)。革兰阳性菌中居前5位的细菌分别为屎肠球菌(30 595株,8.1%)、粪肠球菌(25 056株,6.6%)、表皮葡萄球菌(7 335株,1.9%)、金黄色葡萄球菌(7 125株,1.9%)和溶血葡萄球菌(5 155株,1.4%),见表 1。其中男性患者尿标本分离细菌居前5位的细菌分别是大肠埃希菌、屎肠球菌、粪肠球菌、肺炎克雷伯菌、铜绿假单胞菌,女性患者尿标本分离细菌居前5位的细菌分别是大肠埃希菌、肺炎克雷伯菌、屎肠球菌、粪肠球菌、奇异变形杆菌,见表 2。男性患者尿标本中大肠埃希菌的占比(34.8%)低于女性患者(57.2%),差异具有统计学意义(χ2=18 162.1,P<0.001)。成年患者尿标本分离居前5位的细菌分别是大肠埃希菌、肺炎克雷伯菌、屎肠球菌、粪肠球菌、奇异变形杆菌,儿童患者尿标本分离居前5位的细菌分别是大肠埃希菌、粪肠球菌、屎肠球菌、肺炎克雷伯菌、表皮葡萄球菌,见表 3。成年患者尿标本中大肠埃希菌的占比(49.0%)高于儿童患者(34.4%),差异具有统计学意义(χ2=1 982.1,P<0.001)。
表1(Table 1)
|
表 1 2012—2021年湖南省细菌耐药监测网尿标本分离主要细菌构成情况
Table 1
Constituent of the major bacteria isolated from urine specimens, Hunan Province Antimicrobial Resistance Survei-llance System, 2012-2021
|
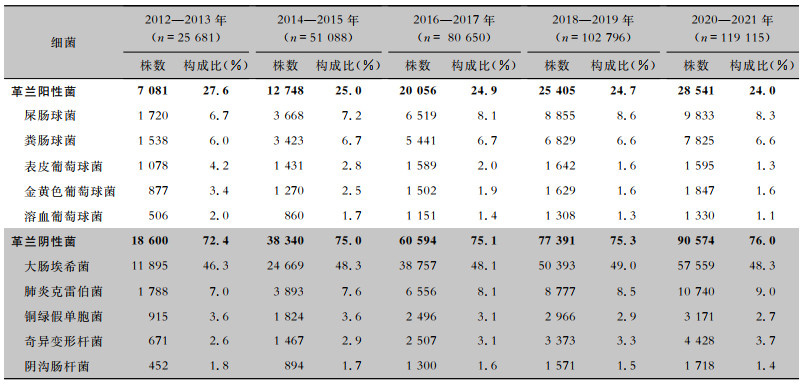
表 1 2012—2021年湖南省细菌耐药监测网尿标本分离主要细菌构成情况
Table 1
Constituent of the major bacteria isolated from urine specimens, Hunan Province Antimicrobial Resistance Survei-llance System, 2012-2021
| 细菌 |
2012—2013年(n=25 681) |
|
2014—2015年(n=51 088) |
|
2016—2017年(n= 80 650) |
|
2018—2019年(n=102 796) |
|
2020—2021年(n=119 115) |
| 株数 |
构成比(%) |
株数 |
构成比(%) |
株数 |
构成比(%) |
株数 |
构成比(%) |
株数 |
构成比(%) |
| 革兰阳性菌 |
7 081 |
27.6 |
|
12 748 |
25.0 |
|
20 056 |
24.9 |
|
25 405 |
24.7 |
|
28 541 |
24.0 |
| 屎肠球菌 |
1 720 |
6.7 |
|
3 668 |
7.2 |
|
6 519 |
8.1 |
|
8 855 |
8.6 |
|
9 833 |
8.3 |
| 粪肠球菌 |
1 538 |
6.0 |
|
3 423 |
6.7 |
|
5 441 |
6.7 |
|
6 829 |
6.6 |
|
7 825 |
6.6 |
| 表皮葡萄球菌 |
1 078 |
4.2 |
|
1 431 |
2.8 |
|
1 589 |
2.0 |
|
1 642 |
1.6 |
|
1 595 |
1.3 |
| 金黄色葡萄球菌 |
877 |
3.4 |
|
1 270 |
2.5 |
|
1 502 |
1.9 |
|
1 629 |
1.6 |
|
1 847 |
1.6 |
| 溶血葡萄球菌 |
506 |
2.0 |
|
860 |
1.7 |
|
1 151 |
1.4 |
|
1 308 |
1.3 |
|
1 330 |
1.1 |
| 革兰阴性菌 |
18 600 |
72.4 |
|
38 340 |
75.0 |
|
60 594 |
75.1 |
|
77 391 |
75.3 |
|
90 574 |
76.0 |
| 大肠埃希菌 |
11 895 |
46.3 |
|
24 669 |
48.3 |
|
38 757 |
48.1 |
|
50 393 |
49.0 |
|
57 559 |
48.3 |
| 肺炎克雷伯菌 |
1 788 |
7.0 |
|
3 893 |
7.6 |
|
6 556 |
8.1 |
|
8 777 |
8.5 |
|
10 740 |
9.0 |
| 铜绿假单胞菌 |
915 |
3.6 |
|
1 824 |
3.6 |
|
2 496 |
3.1 |
|
2 966 |
2.9 |
|
3 171 |
2.7 |
| 奇异变形杆菌 |
671 |
2.6 |
|
1 467 |
2.9 |
|
2 507 |
3.1 |
|
3 373 |
3.3 |
|
4 428 |
3.7 |
| 阴沟肠杆菌 |
452 |
1.8 |
|
894 |
1.7 |
|
1 300 |
1.6 |
|
1 571 |
1.5 |
|
1 718 |
1.4 |
|
表2(Table 2)
|
表 2 2012—2021年湖南省细菌耐药监测网不同性别患者尿标本分离主要菌株构成情况
Table 2
Constituent of the major bacteria isolated from urine specimens from patients of different genders, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
|
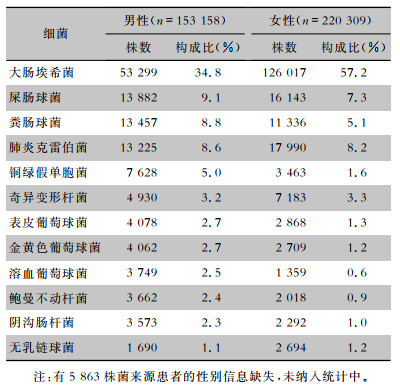
表 2 2012—2021年湖南省细菌耐药监测网不同性别患者尿标本分离主要菌株构成情况
Table 2
Constituent of the major bacteria isolated from urine specimens from patients of different genders, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
| 细菌 |
男性(n=153 158) |
|
女性(n=220 309) |
| 株数 |
构成比(%) |
株数 |
构成比(%) |
| 大肠埃希菌 |
53 299 |
34.8 |
|
126 017 |
57.2 |
| 屎肠球菌 |
13 882 |
9.1 |
|
16 143 |
7.3 |
| 粪肠球菌 |
13 457 |
8.8 |
|
11 336 |
5.1 |
| 肺炎克雷伯菌 |
13 225 |
8.6 |
|
17 990 |
8.2 |
| 铜绿假单胞菌 |
7 628 |
5.0 |
|
3 463 |
1.6 |
| 奇异变形杆菌 |
4 930 |
3.2 |
|
7 183 |
3.3 |
| 表皮葡萄球菌 |
4 078 |
2.7 |
|
2 868 |
1.3 |
| 金黄色葡萄球菌 |
4 062 |
2.7 |
|
2 709 |
1.2 |
| 溶血葡萄球菌 |
3 749 |
2.5 |
|
1 359 |
0.6 |
| 鲍曼不动杆菌 |
3 662 |
2.4 |
|
2 018 |
0.9 |
| 阴沟肠杆菌 |
3 573 |
2.3 |
|
2 292 |
1.0 |
| 无乳链球菌 |
1 690 |
1.1 |
|
2 694 |
1.2 |
| 注:有5 863株菌来源患者的性别信息缺失,未纳入统计中。 |
|
表3(Table 3)
|
表 3 2012—2021年湖南省细菌耐药监测网不同年龄患者尿标本分离主要菌株构成情况
Table 3
Constituent of the major bacteria isolated from urine specimens from patients of different ages, Hunan Province Antimicrobial Resistance Survei-llance System, 2012-2021
|
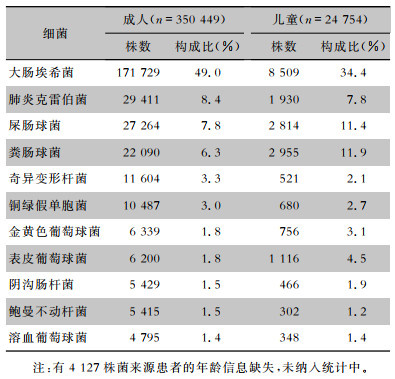
表 3 2012—2021年湖南省细菌耐药监测网不同年龄患者尿标本分离主要菌株构成情况
Table 3
Constituent of the major bacteria isolated from urine specimens from patients of different ages, Hunan Province Antimicrobial Resistance Survei-llance System, 2012-2021
| 细菌 |
成人(n=350 449) |
|
儿童(n=24 754) |
| 株数 |
构成比(%) |
株数 |
构成比(%) |
| 大肠埃希菌 |
171 729 |
49.0 |
|
8 509 |
34.4 |
| 肺炎克雷伯菌 |
29 411 |
8.4 |
|
1 930 |
7.8 |
| 屎肠球菌 |
27 264 |
7.8 |
|
2 814 |
11.4 |
| 粪肠球菌 |
22 090 |
6.3 |
|
2 955 |
11.9 |
| 奇异变形杆菌 |
11 604 |
3.3 |
|
521 |
2.1 |
| 铜绿假单胞菌 |
10 487 |
3.0 |
|
680 |
2.7 |
| 金黄色葡萄球菌 |
6 339 |
1.8 |
|
756 |
3.1 |
| 表皮葡萄球菌 |
6 200 |
1.8 |
|
1 116 |
4.5 |
| 阴沟肠杆菌 |
5 429 |
1.5 |
|
466 |
1.9 |
| 鲍曼不动杆菌 |
5 415 |
1.5 |
|
302 |
1.2 |
| 溶血葡萄球菌 |
4 795 |
1.4 |
|
348 |
1.4 |
| 注:有4 127株菌来源患者的年龄信息缺失,未纳入统计中。 |
|
2.2 主要分离菌的药敏情况
2.2.1 大肠埃希菌
2012—2021年从尿标本中分离的大肠埃希菌对碳青霉烯类抗生素、阿米卡星、替加环素、头孢哌酮/舒巴坦、呋喃妥因保持较高的敏感性,耐药率<10%;对头孢唑林、头孢呋辛、头孢曲松、头孢噻肟、喹诺酮类抗菌药物的耐药性较高,耐药率>48%。对头孢他啶、头孢曲松、头孢噻肟、头孢吡肟、氨曲南的耐药率呈下降趋势(χ2趋势分别为1 062.9、1 032.2、218.0、2 664.3、1 361.0,均P<0.001)。见表 4。
表4(Table 4)
|
表 4 2012—2021年湖南省细菌耐药监测网尿标本分离大肠埃希菌对抗菌药物的药敏结果
Table 4
Antimicrobial susceptibility testing results of Escherichia coli isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
|
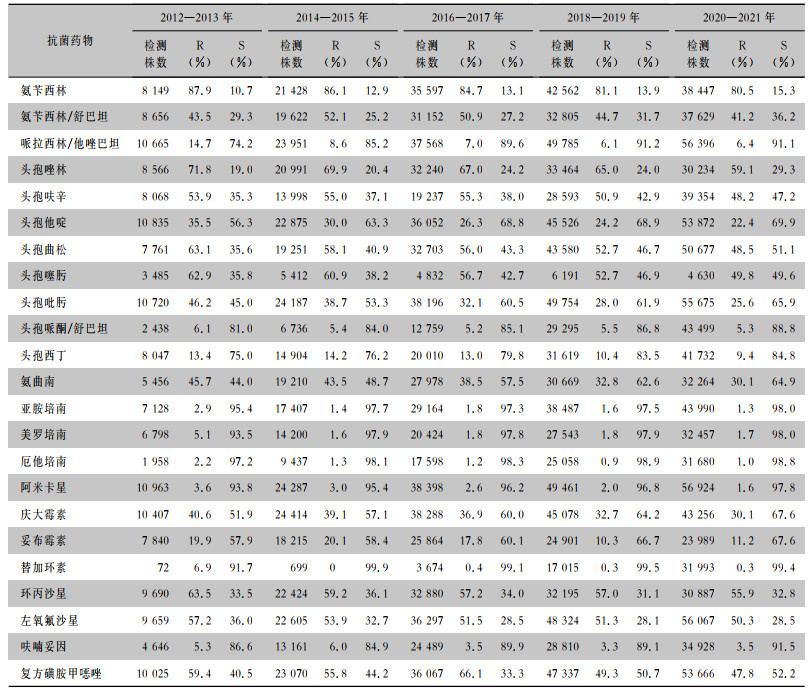
表 4 2012—2021年湖南省细菌耐药监测网尿标本分离大肠埃希菌对抗菌药物的药敏结果
Table 4
Antimicrobial susceptibility testing results of Escherichia coli isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
| 抗菌药物 |
2012—2013 |
|
2014—2015 |
|
2016—2017 |
|
2018—2019 |
|
2020—2021 |
| 检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
| 氨苄西林 |
8 149 |
87.9 |
10.7 |
|
21 428 |
86.1 |
12.9 |
|
35 597 |
84.7 |
13.1 |
|
42 562 |
81.1 |
13.9 |
|
38 447 |
80.5 |
15.3 |
| 氨苄西林/舒巴坦 |
8 656 |
43.5 |
29.3 |
|
19 622 |
52.1 |
25.2 |
|
31 152 |
50.9 |
27.2 |
|
32 805 |
44.7 |
31.7 |
|
37 629 |
41.2 |
36.2 |
| 哌拉西林/他唑巴坦 |
10 665 |
14.7 |
74.2 |
|
23 951 |
8.6 |
85.2 |
|
37 568 |
7.0 |
89.6 |
|
49 785 |
6.1 |
91.2 |
|
56 396 |
6.4 |
91.1 |
| 头孢唑林 |
8 566 |
71.8 |
19.0 |
|
20 991 |
69.9 |
20.4 |
|
32 240 |
67.0 |
24.2 |
|
33 464 |
65.0 |
24.0 |
|
30 234 |
59.1 |
29.3 |
| 头孢呋辛 |
8 068 |
53.9 |
35.3 |
|
13 998 |
55.0 |
37.1 |
|
19 237 |
55.3 |
38.0 |
|
28 593 |
50.9 |
42.9 |
|
39 354 |
48.2 |
47.2 |
| 头孢他啶 |
10 835 |
35.5 |
56.3 |
|
22 875 |
30.0 |
63.3 |
|
36 052 |
26.3 |
68.8 |
|
45 526 |
24.2 |
68.9 |
|
53 872 |
22.4 |
69.9 |
| 头孢曲松 |
7 761 |
63.1 |
35.6 |
|
19 251 |
58.1 |
40.9 |
|
32 703 |
56.0 |
43.3 |
|
43 580 |
52.7 |
46.7 |
|
50 677 |
48.5 |
51.1 |
| 头孢噻肟 |
3 485 |
62.9 |
35.8 |
|
5 412 |
60.9 |
38.2 |
|
4 832 |
56.7 |
42.7 |
|
6 191 |
52.7 |
46.9 |
|
4 630 |
49.8 |
49.6 |
| 头孢吡肟 |
10 720 |
46.2 |
45.0 |
|
24 187 |
38.7 |
53.3 |
|
38 196 |
32.1 |
60.5 |
|
49 754 |
28.0 |
61.9 |
|
55 675 |
25.6 |
65.9 |
| 头孢哌酮/舒巴坦 |
2 438 |
6.1 |
81.0 |
|
6 736 |
5.4 |
84.0 |
|
12 759 |
5.2 |
85.1 |
|
29 295 |
5.5 |
86.8 |
|
43 499 |
5.3 |
88.8 |
| 头孢西丁 |
8 047 |
13.4 |
75.0 |
|
14 904 |
14.2 |
76.2 |
|
20 010 |
13.0 |
79.8 |
|
31 619 |
10.4 |
83.5 |
|
41 732 |
9.4 |
84.8 |
| 氨曲南 |
5 456 |
45.7 |
44.0 |
|
19 210 |
43.5 |
48.7 |
|
27 978 |
38.5 |
57.5 |
|
30 669 |
32.8 |
62.6 |
|
32 264 |
30.1 |
64.9 |
| 亚胺培南 |
7 128 |
2.9 |
95.4 |
|
17 407 |
1.4 |
97.7 |
|
29 164 |
1.8 |
97.3 |
|
38 487 |
1.6 |
97.5 |
|
43 990 |
1.3 |
98.0 |
| 美罗培南 |
6 798 |
5.1 |
93.5 |
|
14 200 |
1.6 |
97.9 |
|
20 424 |
1.8 |
97.8 |
|
27 543 |
1.8 |
97.9 |
|
32 457 |
1.7 |
98.0 |
| 厄他培南 |
1 958 |
2.2 |
97.2 |
|
9 437 |
1.3 |
98.1 |
|
17 598 |
1.2 |
98.3 |
|
25 058 |
0.9 |
98.9 |
|
31 680 |
1.0 |
98.8 |
| 阿米卡星 |
10 963 |
3.6 |
93.8 |
|
24 287 |
3.0 |
95.4 |
|
38 398 |
2.6 |
96.2 |
|
49 461 |
2.0 |
96.8 |
|
56 924 |
1.6 |
97.8 |
| 庆大霉素 |
10 407 |
40.6 |
51.9 |
|
24 414 |
39.1 |
57.1 |
|
38 288 |
36.9 |
60.0 |
|
45 078 |
32.7 |
64.2 |
|
43 256 |
30.1 |
67.6 |
| 妥布霉素 |
7 840 |
19.9 |
57.9 |
|
18 215 |
20.1 |
58.4 |
|
25 864 |
17.8 |
60.1 |
|
24 901 |
10.3 |
66.7 |
|
23 989 |
11.2 |
67.6 |
| 替加环素 |
72 |
6.9 |
91.7 |
|
699 |
0 |
99.9 |
|
3 674 |
0.4 |
99.1 |
|
17 015 |
0.3 |
99.5 |
|
31 993 |
0.3 |
99.4 |
| 环丙沙星 |
9 690 |
63.5 |
33.5 |
|
22 424 |
59.2 |
36.1 |
|
32 880 |
57.2 |
34.0 |
|
32 195 |
57.0 |
31.1 |
|
30 887 |
55.9 |
32.8 |
| 左氧氟沙星 |
9 659 |
57.2 |
36.0 |
|
22 605 |
53.9 |
32.7 |
|
36 297 |
51.5 |
28.5 |
|
48 324 |
51.3 |
28.1 |
|
56 067 |
50.3 |
28.5 |
| 呋喃妥因 |
4 646 |
5.3 |
86.6 |
|
13 161 |
6.0 |
84.9 |
|
24 489 |
3.5 |
89.9 |
|
28 810 |
3.3 |
89.1 |
|
34 928 |
3.5 |
91.5 |
复方磺胺甲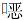 唑 唑 |
10 025 |
59.4 |
40.5 |
|
23 070 |
55.8 |
44.2 |
|
36 067 |
66.1 |
33.3 |
|
47 337 |
49.3 |
50.7 |
|
53 666 |
47.8 |
52.2 |
|
2.2.2 肠球菌
2012—2021年屎肠球菌、粪肠球菌对万古霉素、替考拉宁、利奈唑胺均保持较高的敏感性,耐药率均<5%。粪肠球菌对氨苄西林、呋喃妥因的耐药率<15%。除利奈唑胺和米诺环素外,屎肠球菌对检测抗菌药物的耐药率均高于粪肠球菌。见表 5、6。
表5(Table 5)
|
表 5 2012—2021年湖南省细菌耐药监测网尿标本分离屎肠球菌对抗菌药物的药敏结果
Table 5
Antimicrobial susceptibility testing results of Enterococcus faecium isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
|
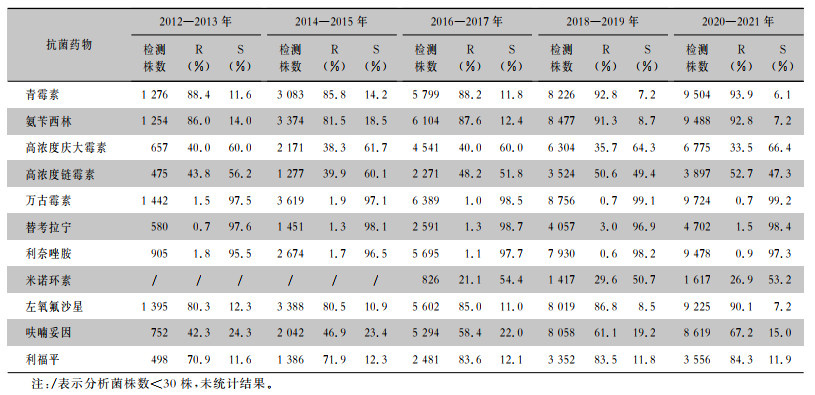
表 5 2012—2021年湖南省细菌耐药监测网尿标本分离屎肠球菌对抗菌药物的药敏结果
Table 5
Antimicrobial susceptibility testing results of Enterococcus faecium isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
| 抗菌药物 |
2012—2013 |
|
2014—2015 |
|
2016—2017 |
|
2018—2019 |
|
2020—2021 |
| 检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
| 青霉素 |
1 276 |
88.4 |
11.6 |
|
3 083 |
85.8 |
14.2 |
|
5 799 |
88.2 |
11.8 |
|
8 226 |
92.8 |
7.2 |
|
9 504 |
93.9 |
6.1 |
| 氨苄西林 |
1 254 |
86.0 |
14.0 |
|
3 374 |
81.5 |
18.5 |
|
6 104 |
87.6 |
12.4 |
|
8 477 |
91.3 |
8.7 |
|
9 488 |
92.8 |
7.2 |
| 高浓度庆大霉素 |
657 |
40.0 |
60.0 |
|
2 171 |
38.3 |
61.7 |
|
4 541 |
40.0 |
60.0 |
|
6 304 |
35.7 |
64.3 |
|
6 775 |
33.5 |
66.4 |
| 高浓度链霉素 |
475 |
43.8 |
56.2 |
|
1 277 |
39.9 |
60.1 |
|
2 271 |
48.2 |
51.8 |
|
3 524 |
50.6 |
49.4 |
|
3 897 |
52.7 |
47.3 |
| 万古霉素 |
1 442 |
1.5 |
97.5 |
|
3 619 |
1.9 |
97.1 |
|
6 389 |
1.0 |
98.5 |
|
8 756 |
0.7 |
99.1 |
|
9 724 |
0.7 |
99.2 |
| 替考拉宁 |
580 |
0.7 |
97.6 |
|
1 451 |
1.3 |
98.1 |
|
2 591 |
1.3 |
98.7 |
|
4 057 |
3.0 |
96.9 |
|
4 702 |
1.5 |
98.4 |
| 利奈唑胺 |
905 |
1.8 |
95.5 |
|
2 674 |
1.7 |
96.5 |
|
5 695 |
1.1 |
97.7 |
|
7 930 |
0.6 |
98.2 |
|
9 478 |
0.9 |
97.3 |
| 米诺环素 |
/ |
/ |
/ |
|
/ |
/ |
/ |
|
826 |
21.1 |
54.4 |
|
1 417 |
29.6 |
50.7 |
|
1 617 |
26.9 |
53.2 |
| 左氧氟沙星 |
1 395 |
80.3 |
12.3 |
|
3 388 |
80.5 |
10.9 |
|
5 602 |
85.0 |
11.0 |
|
8 019 |
86.8 |
8.5 |
|
9 225 |
90.1 |
7.2 |
| 呋喃妥因 |
752 |
42.3 |
24.3 |
|
2 042 |
46.9 |
23.4 |
|
5 294 |
58.4 |
22.0 |
|
8 058 |
61.1 |
19.2 |
|
8 619 |
67.2 |
15.0 |
| 利福平 |
498 |
70.9 |
11.6 |
|
1 386 |
71.9 |
12.3 |
|
2 481 |
83.6 |
12.1 |
|
3 352 |
83.5 |
11.8 |
|
3 556 |
84.3 |
11.9 |
| 注:/表示分析菌株数<30株,未统计结果。 |
|
表6(Table 6)
|
表 6 2012—2021年湖南省细菌耐药监测网尿标本分离粪肠球菌对抗菌药物的药敏结果
Table 6
Antimicrobial susceptibility testing results of Enterococcus faecalis isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
|
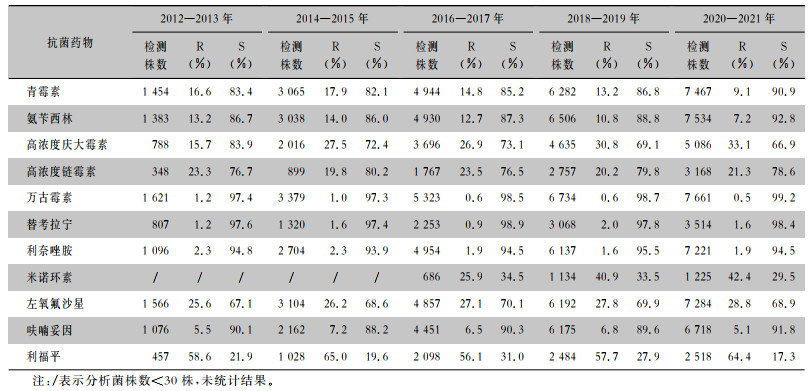
表 6 2012—2021年湖南省细菌耐药监测网尿标本分离粪肠球菌对抗菌药物的药敏结果
Table 6
Antimicrobial susceptibility testing results of Enterococcus faecalis isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
| 抗菌药物 |
2012—2013 |
|
2014—2015 |
|
2016—2017 |
|
2018—2019 |
|
2020—2021 |
| 检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
| 青霉素 |
1 454 |
16.6 |
83.4 |
|
3 065 |
17.9 |
82.1 |
|
4 944 |
14.8 |
85.2 |
|
6 282 |
13.2 |
86.8 |
|
7 467 |
9.1 |
90.9 |
| 氨苄西林 |
1 383 |
13.2 |
86.7 |
|
3 038 |
14.0 |
86.0 |
|
4 930 |
12.7 |
87.3 |
|
6 506 |
10.8 |
88.8 |
|
7 534 |
7.2 |
92.8 |
| 高浓度庆大霉素 |
788 |
15.7 |
83.9 |
|
2 016 |
27.5 |
72.4 |
|
3 696 |
26.9 |
73.1 |
|
4 635 |
30.8 |
69.1 |
|
5 086 |
33.1 |
66.9 |
| 高浓度链霉素 |
348 |
23.3 |
76.7 |
|
899 |
19.8 |
80.2 |
|
1 767 |
23.5 |
76.5 |
|
2 757 |
20.2 |
79.8 |
|
3 168 |
21.3 |
78.6 |
| 万古霉素 |
1 621 |
1.2 |
97.4 |
|
3 379 |
1.0 |
97.3 |
|
5 323 |
0.6 |
98.5 |
|
6 734 |
0.6 |
98.7 |
|
7 661 |
0.5 |
99.2 |
| 替考拉宁 |
807 |
1.2 |
97.6 |
|
1 320 |
1.6 |
97.4 |
|
2 253 |
0.9 |
98.9 |
|
3 068 |
2.0 |
97.8 |
|
3 514 |
1.6 |
98.4 |
| 利奈唑胺 |
1 096 |
2.3 |
94.8 |
|
2 704 |
2.3 |
93.9 |
|
4 954 |
1.9 |
94.5 |
|
6 137 |
1.6 |
95.5 |
|
7 221 |
1.9 |
94.5 |
| 米诺环素 |
/ |
/ |
/ |
|
/ |
/ |
/ |
|
686 |
25.9 |
34.5 |
|
1 134 |
40.9 |
33.5 |
|
1 225 |
42.4 |
29.5 |
| 左氧氟沙星 |
1 566 |
25.6 |
67.1 |
|
3 104 |
26.2 |
68.6 |
|
4 857 |
27.1 |
70.1 |
|
6 192 |
27.8 |
69.9 |
|
7 284 |
28.8 |
68.9 |
| 呋喃妥因 |
1 076 |
5.5 |
90.1 |
|
2 162 |
7.2 |
88.2 |
|
4 451 |
6.5 |
90.3 |
|
6 175 |
6.8 |
89.6 |
|
6 718 |
5.1 |
91.8 |
| 利福平 |
457 |
58.6 |
21.9 |
|
1 028 |
65.0 |
19.6 |
|
2 098 |
56.1 |
31.0 |
|
2 484 |
57.7 |
27.9 |
|
2 518 |
64.4 |
17.3 |
| 注:/表示分析菌株数<30株,未统计结果。 |
|
2.2.3 肺炎克雷伯菌
2012—2021年从尿标本分离的肺炎克雷伯菌对碳青霉烯类抗生素、阿米卡星、替加环素保持较高的敏感性,耐药率<11%,对喹诺酮类药物耐药率远低于大肠埃希菌。见表 7。
表7(Table 7)
|
表 7 2012—2021年湖南省细菌耐药监测网尿标本分离肺炎克雷伯菌对抗菌药物的药敏结果
Table 7
Antimicrobial susceptibility testing results of Klebsiella pneumoniae isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
|
表 7 2012—2021年湖南省细菌耐药监测网尿标本分离肺炎克雷伯菌对抗菌药物的药敏结果
Table 7
Antimicrobial susceptibility testing results of Klebsiella pneumoniae isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
| 抗菌药物 |
2012—2013 |
|
2014—2015 |
|
2016—2017 |
|
2018—2019 |
|
2020—2021 |
| 检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
| 氨苄西林/舒巴坦 |
1 381 |
50.0 |
35.9 |
|
3 195 |
51.6 |
38.1 |
|
5 103 |
49.8 |
39.9 |
|
5 440 |
45.7 |
44.1 |
|
6 995 |
43.5 |
46.9 |
| 哌拉西林/他唑巴坦 |
1 618 |
23.4 |
68.7 |
|
3 775 |
18.0 |
77.9 |
|
6 327 |
18.4 |
77.8 |
|
8 684 |
18.9 |
77.1 |
|
10 606 |
19.3 |
75.9 |
| 头孢唑林 |
1 355 |
61.1 |
28.8 |
|
3 375 |
58.5 |
30.4 |
|
5 542 |
58.5 |
33.8 |
|
5 856 |
58.1 |
35.5 |
|
5 861 |
51.0 |
42.8 |
| 头孢呋辛 |
1 152 |
51.6 |
40.3 |
|
2 027 |
49.3 |
45.0 |
|
3 332 |
50.3 |
44.4 |
|
5 044 |
46.6 |
49.4 |
|
7 423 |
42.5 |
54.2 |
| 头孢他啶 |
1 636 |
37.9 |
55.6 |
|
3 566 |
31.3 |
64.1 |
|
6 079 |
29.9 |
65.5 |
|
7 897 |
28.0 |
66.8 |
|
9 959 |
27.7 |
67.2 |
| 头孢曲松 |
1 277 |
54.1 |
43.9 |
|
3 132 |
47.5 |
50.7 |
|
5 665 |
47.1 |
52.0 |
|
7 688 |
44.4 |
54.7 |
|
9 407 |
41.0 |
58.3 |
| 头孢噻肟 |
444 |
59.0 |
39.2 |
|
844 |
50.9 |
48.2 |
|
694 |
52.2 |
46.3 |
|
880 |
41.6 |
56.7 |
|
719 |
38.4 |
55.5 |
| 头孢吡肟 |
1 607 |
39.1 |
54.9 |
|
3 813 |
31.3 |
64.8 |
|
6 485 |
29.4 |
65.8 |
|
8 696 |
26.3 |
68.3 |
|
10 450 |
26.2 |
69.1 |
| 头孢哌酮/舒巴坦 |
297 |
10.8 |
70.0 |
|
1 042 |
10.1 |
81.4 |
|
2 155 |
14.5 |
76.6 |
|
5 190 |
15.2 |
77.6 |
|
8 543 |
15.8 |
78.9 |
| 头孢西丁 |
1 161 |
23.4 |
68.3 |
|
2 223 |
24.7 |
69.1 |
|
3 492 |
24.3 |
70.6 |
|
5 605 |
22.0 |
74.2 |
|
7 998 |
20.5 |
76.4 |
| 氨曲南 |
1 378 |
43.4 |
45.8 |
|
3 183 |
38.3 |
53.8 |
|
4 772 |
37.0 |
60.0 |
|
5 587 |
33.9 |
63.0 |
|
6 236 |
32.4 |
65.0 |
| 亚胺培南 |
1 075 |
4.3 |
94.6 |
|
2 920 |
6.6 |
91.0 |
|
4 784 |
7.6 |
89.9 |
|
6 553 |
8.1 |
89.1 |
|
8 054 |
8.7 |
89.1 |
| 美罗培南 |
1 007 |
6.1 |
92.5 |
|
2 206 |
5.0 |
94.3 |
|
3 609 |
8.9 |
90.3 |
|
4 894 |
8.9 |
90.0 |
|
6 482 |
10.4 |
88.6 |
| 厄他培南 |
354 |
3.4 |
95.8 |
|
1 622 |
8.5 |
90.4 |
|
2 844 |
6.9 |
92.7 |
|
4 195 |
7.1 |
92.2 |
|
5 561 |
8.6 |
90.7 |
| 阿米卡星 |
1 653 |
6.4 |
91.0 |
|
3 827 |
7.9 |
91.0 |
|
6 506 |
8.4 |
91.0 |
|
8 651 |
8.4 |
91.0 |
|
10 599 |
8.3 |
91.3 |
| 庆大霉素 |
1 556 |
33.3 |
61.6 |
|
3 858 |
31.3 |
65.8 |
|
6 465 |
29.5 |
68.4 |
|
7 770 |
25.1 |
72.3 |
|
8 074 |
23.5 |
74.6 |
| 妥布霉素 |
1 240 |
16.3 |
67.1 |
|
2 811 |
19.5 |
65.0 |
|
4 042 |
18.1 |
65.5 |
|
4 218 |
13.1 |
71.2 |
|
4 494 |
15.3 |
70.2 |
| 替加环素 |
/ |
/ |
/ |
|
128 |
4.7 |
93.8 |
|
785 |
2.9 |
91.0 |
|
3 366 |
4.1 |
91.6 |
|
6 466 |
4.8 |
91.3 |
| 环丙沙星 |
/ |
/ |
/ |
|
3 502 |
44.0 |
49.5 |
|
5 214 |
44.2 |
48.3 |
|
5 536 |
45.3 |
45.6 |
|
5 717 |
44.4 |
47.2 |
| 左氧氟沙星 |
1 516 |
33.7 |
59.6 |
|
3 704 |
31.8 |
54.4 |
|
6 146 |
34.0 |
51.1 |
|
8 536 |
34.2 |
49.9 |
|
10 557 |
33.4 |
50.7 |
| 呋喃妥因 |
737 |
29.6 |
39.9 |
|
2 221 |
39.1 |
27.0 |
|
3 980 |
29.4 |
31.6 |
|
4 888 |
33.9 |
31.6 |
|
6 559 |
35.2 |
38.8 |
复方磺胺甲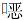 唑 唑 |
1 583 |
52.4 |
47.6 |
|
3 775 |
46.9 |
52.8 |
|
6 252 |
43.2 |
56.6 |
|
8 384 |
41.4 |
58.5 |
|
10 163 |
40.0 |
60.0 |
| 注:/表示分析菌株数<30株,未统计结果。 |
|
2.2.4 金黄色葡萄球菌
2012—2021年从尿标本中分离的金黄色葡萄球菌对万古霉素、利奈唑胺、替考拉宁的耐药率为0,对呋喃妥因保持较高的敏感性,耐药率<10%,对苯唑西林的耐药率维持在28%以上,见表 8。
表8(Table 8)
|
表 8 2012—2021年湖南省细菌耐药监测网尿标本分离金黄色葡萄球菌的药敏结果
Table 8
Antimicrobial susceptibility testing results of Staphylococcus aureus isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
|
表 8 2012—2021年湖南省细菌耐药监测网尿标本分离金黄色葡萄球菌的药敏结果
Table 8
Antimicrobial susceptibility testing results of Staphylococcus aureus isolated from urine specimens, Hunan Province Antimicrobial Resistance Surveillance System, 2012-2021
| 抗菌药物 |
2012—2013 |
|
2014—2015 |
|
2016—2017 |
|
2018—2019 |
|
2020—2021 |
| 检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
检测株数 |
R(%) |
S(%) |
| 青霉素G |
814 |
93.9 |
6.1 |
|
1 149 |
91.6 |
8.4 |
|
1 428 |
91.0 |
9.0 |
|
1 500 |
90.1 |
9.9 |
|
1 727 |
88.8 |
11.1 |
| 苯唑西林 |
804 |
36.7 |
63.3 |
|
1 108 |
43.6 |
56.4 |
|
1 423 |
33.6 |
66.4 |
|
1 510 |
34.9 |
65.1 |
|
1 734 |
28.9 |
71.1 |
| 阿米卡星 |
453 |
10.8 |
83.4 |
|
479 |
5.8 |
90.0 |
|
340 |
5.9 |
92.4 |
|
272 |
9.6 |
89.0 |
|
261 |
6.1 |
92.3 |
| 庆大霉素 |
807 |
23.8 |
68.3 |
|
1 224 |
25.2 |
68.4 |
|
1 483 |
22.1 |
75.2 |
|
1 608 |
18.2 |
77.7 |
|
1 828 |
14.0 |
82.5 |
| 万古霉素 |
841 |
0 |
100 |
|
1 150 |
0 |
100 |
|
1 447 |
0 |
100 |
|
1 584 |
0 |
100 |
|
1 798 |
0 |
100 |
| 替考拉宁 |
132 |
0 |
100 |
|
219 |
0 |
100 |
|
618 |
0 |
100 |
|
733 |
0 |
100 |
|
897 |
0 |
100 |
| 利奈唑胺 |
732 |
0 |
100 |
|
1 080 |
0 |
100 |
|
1 411 |
0 |
100 |
|
1 559 |
0 |
100 |
|
1 804 |
0 |
100 |
| 红霉素 |
842 |
70.7 |
23.6 |
|
947 |
64.4 |
30.5 |
|
1 209 |
61.6 |
35.1 |
|
1 391 |
59.5 |
38.6 |
|
1 559 |
47.9 |
49.7 |
| 克林霉素 |
827 |
42.3 |
50.3 |
|
1 092 |
47.1 |
49.4 |
|
1 367 |
44.9 |
52.8 |
|
1 419 |
36.5 |
60.5 |
|
1 615 |
29.1 |
68.6 |
| 左氧氟沙星 |
669 |
23.6 |
67.1 |
|
881 |
36.4 |
58.3 |
|
1 070 |
29.9 |
67.9 |
|
1 235 |
26.0 |
71.3 |
|
1 576 |
22.0 |
75.9 |
| 呋喃妥因 |
197 |
3.6 |
94.4 |
|
706 |
5.7 |
90.4 |
|
1 050 |
1.8 |
96.8 |
|
1 186 |
2.4 |
96.5 |
|
1 419 |
1.5 |
97.7 |
复方磺胺甲 唑 唑 |
793 |
49.6 |
50.4 |
|
1 205 |
30.9 |
69.0 |
|
1 453 |
19.6 |
80.3 |
|
1 557 |
13.2 |
86.7 |
|
1 743 |
9.9 |
90.1 |
| 利福平 |
816 |
10.4 |
83.7 |
|
1 133 |
16.7 |
78.9 |
|
1 487 |
10.3 |
87.5 |
|
1 602 |
10.1 |
87.2 |
|
1 802 |
6.3 |
92.1 |
|
2.3 重要耐药菌检出率变化趋势
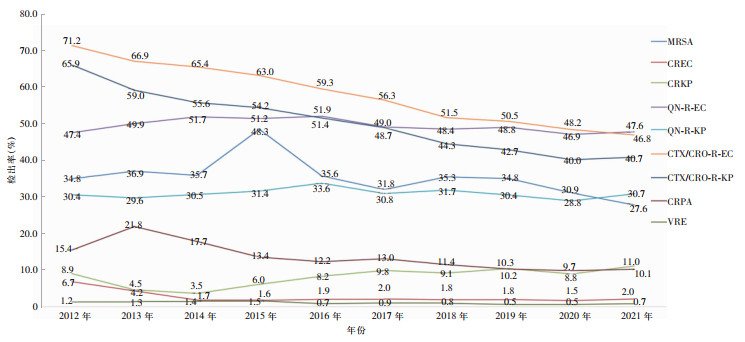
2012—2021年从尿标本中分离出的耐头孢噻肟/头孢曲松大肠埃希菌(CTX/CRO-R-EC)检出率从71.2%下降至46.8%,呈下降趋势(χ2趋势=1 994.0,P<0.001),耐头孢噻肟/头孢曲松肺炎克雷伯菌(CTX/CRO-R-KP) 检出率从65.9%下降至40.7%,耐碳青霉烯类大肠埃希菌(CREC)检出率从6.7%下降至2.0%,耐碳青霉烯类铜绿假单胞菌(CRPA)检出率从15.4%下降至10.1%,耐碳青霉烯类肺炎克雷伯菌(CRKP)检出率从8.9%上升至11.0%,差异均有统计学意义(χ2趋势分别为432.1、165.3、66.0、92.2,均P<0.001)。耐喹诺酮类大肠埃希菌和肺炎克雷伯菌、耐甲氧西林金黄色葡萄球菌、耐万古霉素肠球菌检出率10年间变化不大。见图 2。
3 讨论
本组监测结果显示,2012—2021年湖南省细菌耐药监测网成员单位尿标本检出细菌数量呈逐年上升趋势。菌株分布主要为革兰阴性菌,革兰阴性菌中以大肠埃希菌为主,革兰阳性菌中以屎肠球菌和粪肠球菌为主,与既往全国及地区尿标本来源细菌分布一致[12-15]。不同性别患者尿标本病原菌构成不同,虽然男性患者和女性患者尿标本检出细菌前4位均为大肠埃希菌、屎肠球菌、粪肠球菌、肺炎克雷伯菌,但是所占比例不同,女性患者尿标本大肠埃希菌占比>50%,而在男性患者尿标本中仅占35%左右,与既往CARSS[12]数据一致。不同年龄段患者尿标本病原菌构成不同,大肠埃希菌在成年和儿童患者尿标本中虽然都是第一位的病原菌,但在成年患者中所占比率(49.0%)高于儿童患者(34.4%)。
目前,肠球菌属已成为尿路感染的重要致病菌。本次监测中,屎肠球菌和粪肠球菌对万古霉素、替考拉宁和利奈唑胺均保持较高的敏感性(敏感率>95%),粪肠球菌对氨苄西林、呋喃妥因的耐药率较低(<15%),且10年间耐药率变化不大。除利奈唑胺和米诺环素,屎肠球菌对检测抗菌药物的耐药率均高于粪肠球菌,与既往全国报道[12, 16]一致。本次监测未发现对万古霉素、利奈唑胺、替考拉宁耐药的金黄色葡萄球菌。
湖南省细菌耐药监测网监测数据显示,10年间尿标本分离的大肠埃希菌达40%以上,对碳青霉烯类抗生素、阿米卡星、替加环素、头孢哌酮/舒巴坦、呋喃妥因保持较高的敏感性,耐药率<10%,与相关学者[13, 15]报道的结果一致,这些药物可根据药敏试验结果用于大肠埃希菌引起的尿路感染。检出的大肠埃希菌对头孢他啶、头孢曲松、头孢噻肟、头孢吡肟、氨曲南的耐药率呈下降趋势, 提示做好细菌耐药性监测,规范抗感染治疗,对控制细菌耐药具有重要意义。各年度大肠埃希菌对喹诺酮类抗菌药物的耐药率>50%,可能与临床习惯经验性使用此类药物治疗泌尿系统感染有关,药物主动外排、药物作用靶位改变、膜通透性降低、质粒基因介导等因素,会诱导该菌对喹诺酮类药物产生耐药性。本次监测中,肺炎克雷伯菌对碳青霉烯类抗生素、阿米卡星、替加环素、头孢哌酮/舒巴坦的耐药率整体上略高于大肠埃希菌,与中国细菌耐药监测网(CHINET)及相关学者[16-17]报道的结果一致。肺炎克雷伯菌对呋喃妥因的耐药率10年间均>25%,高于大肠埃希菌,而对喹诺酮类药物耐药率远低于大肠埃希菌,与相关研究[3, 18-19]结果一致。本次监测中大肠埃希菌对碳青霉烯类抗生素的耐药率呈下降趋势,与其他相关报道大肠埃希菌对碳青霉烯类抗生素的耐药率呈逐年上升趋势[3, 16, 18]不同,说明湖南省各医院对大肠埃希菌引起的尿路感染,在使用碳青霉烯类抗生素抗感染方面把控较为严格,在延缓此类抗生素耐药方面做了许多积极工作。
综上所述,2012—2021年湖南省细菌耐药监测网成员单位尿标本检出细菌以革兰阴性菌为主,随着抗菌药物的广泛使用,尿标本检出的各种细菌对各类抗菌药物呈现不同程度的耐药性。应综合考虑患者的性别、年龄以及地域性差异,同时动态监测尿标本病原菌分布情况及耐药性变化趋势,充分了解尿路感染病原谱变化以及抗菌药物耐药率变迁情况,以更好指导临床合理使用抗菌药物,减少耐药菌株的产生。
利益冲突:所有作者均声明不存在利益冲突。
参考文献
| [1] |
赖蓓, 葛春悦, 宋刚, 等. 2016年至2020年老年患者尿标本分离细菌的耐药性变迁的研究[J]. 中国临床药理学杂志, 2022, 38(24): 3031-3035. Lai B, Ge CY, Song G, et al. Study on bacterial resistance surveillance from urine specimens of geriatric patients from 2016 to 2020[J]. The Chinese Journal of Clinical Pharmacology, 2022, 38(24): 3031-3035. |
| [2] |
Asadi Karam MR, Habibi M, Bouzari S. Urinary tract infection: pathogenicity, antibiotic resistance and development of effective vaccines against uropathogenic Escherichia coli[J]. Mol Immunol, 2019, 108: 56-67. DOI:10.1016/j.molimm.2019.02.007 |
| [3] |
马志刚, 张鸿娟, 董小雪, 等. 2017—2021年滇中及滇西部地区多家三级医院尿液病原菌分布和耐药分析[J]. 国际检验医学杂志, 2023, 44(1): 8-16, 23. Ma ZG, Zhang HJ, Dong XX, et al. Distribution and drug resistance analysis of urinary pathogenic bacteria in several tertiary hospitals in central and western Yunnan from 2017 to 2021[J]. International Journal of Laboratory Medicine, 2023, 44(1): 8-16, 23. |
| [4] | |
| [5] |
Majeed A, Alarfaj S, Darouiche R, et al. An update on emerging therapies for urinary tract infections[J]. Expert Opin Emerg Drugs, 2017, 22(1): 53-62. DOI:10.1080/14728214.2017.1293650 |
| [6] | |
| [7] |
Clinical and Laboratory Standards Institute. M100—Performance standards for antimicrobial susceptibility testing, 32nd edition[S]. Malvern, PA, USA: CLSI, 2022.
|
| [8] |
Barry AL, Jones RN. Criteria for disk susceptibility tests and quality control guidelines for the cefoperazone-sulbactam combination[J]. J Clin Microbiol, 1988, 26(1): 13-17. DOI:10.1128/jcm.26.1.13-17.1988 |
| [9] |
Jones RN, Barry AL, Packer RR, et al. In vitro antimicrobial spectrum, occurrence of synergy, and recommendations for dilution susceptibility testing concentrations of the cefoperazone-sulbactam combination[J]. J Clin Microbiol, 1987, 25(9): 1725-1729. DOI:10.1128/jcm.25.9.1725-1729.1987 |
| [10] | |
| [11] |
Satlin MJ, Lewis JS Ⅱ, Weinstein MP, et al. Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing position statements on polymyxin B and colistin clinical breakpoints[J]. Clin Infect Dis, 2020, 71(9): e523-e529. |
| [12] |
全国细菌耐药监测网. 全国细菌耐药监测网2014—2019年尿标本细菌耐药监测报告[J]. 中国感染控制杂志, 2021, 20(1): 52-59. China Antimicrobial Resistance Surveillance System. Antimicrobial resistance of bacteria from urine specimens: surveillance report from China Antimicrobial Resistance Surveillance System in 2014 -2019[J]. Chinese Journal of Infection Control, 2021, 20(1): 52-59. DOI:10.12138/j.issn.1671-9638.20216181 |
| [13] |
龙姗姗, 喻华, 黄湘宁, 等. 2015—2018年四川省细菌耐药监测网尿液标本细菌分布及耐药性分析[J]. 中华医院感染学杂志, 2020, 30(7): 1066-1071. Long SS, Yu H, Huang XN, et al. Distribution and drug resistance of bacteria in urine samples of Sichuan Provincial Antimicrobial Resistant Investigation Net from 2015 to 2018[J]. Chinese Journal of Nosocomiology, 2020, 30(7): 1066-1071. |
| [14] |
杜雅丽, 胡爱玲, 衡媛, 等. 2018—2021年医院尿培养病原菌及菌株耐药性变迁[J]. 西北药学杂志, 2023, 38(2): 208-211. Du YL, Hu AL, Heng Y, et al. Changes of pathogens and drug resistance of strains in hospital urine culture from 2018 to 2021[J]. Northwest Pharmaceutical Journal, 2023, 38(2): 208-211. |
| [15] |
宿瑞俊, 郑文琪, 申慧敏, 等. 2016—2020年内蒙古某教学医院尿液标本分离菌的耐药性分析及部分大肠埃希菌多位点序列分型[J]. 中国感染与化疗杂志, 2022, 22(5): 597-603.
Su RJ, Zheng WQ, Shen HM, et al. Antimicrobial resistance profile of the bacterial isolates from urine in the period from 2016 to 2020 and multilocus sequence typing of Escherichia coli isolates in a teaching hospital in Inner Mongolia Autonomous Region[J]. Chinese Journal of Infection and Chemotherapy, 2022, 22(5): 597-603. |
| [16] |
胡付品, 郭燕, 朱德妹, 等. 2021年CHINET中国细菌耐药监测[J]. 中国感染与化疗杂志, 2022, 22(5): 521-530. Hu FP, Guo Y, Zhu DM, et al. CHINET surveillance of antimicrobial resistance among the bacterial isolates in 2021[J]. Chinese Journal of Infection and Chemotherapy, 2022, 22(5): 521-530. |
| [17] |
Li J, Jiang FF, Xie A, et al. Analysis of the distribution and drug resistance of pathogens in patients with urinary tract infection in the eastern Chongming area of Shanghai from 2018 to 2020[J]. Infect Drug Resist, 2022, 15: 6413-6422. DOI:10.2147/IDR.S384515 |
| [18] |
赵梅, 杨丹, 贾伟, 等. 2018—2020年多中心尿标本分离菌分布及耐药性分析[J]. 中国抗生素杂志, 2021, 46(11): 1008-1013. Zhao M, Yang D, Jia W, et al. Bacterial distribution and drug resistance of bacteria from urine specimens in a multicenter from 2018 to 2020[J]. Chinese Journal of Antibiotics, 2021, 46(11): 1008-1013. |
| [19] |
Zhang JD, Xie LG, Cao Y, et al. Characteristics of and antibiotic resistance in urinary tract pathogens isolated from patients with upper urinary tract stones[J]. Pak J Pharm Sci, 2023, 36(1): 23-29. |












 唑
唑